Explain Why Water Has Different Properties From Carbon Dioxide
Open stomata allow water vapor to leave the leaf but also allow carbon dioxide CO 2 to enter. Describe how the properties of oxygen carbon dioxide and water contribute to their physiological functions.
Carbon Dioxide Molecule Of The Month May 2012 Html Only Version
When it dissolves it forms a carbonic acid.
. Carbon dioxide molecules cannot form hydrogen bonds with other carbon dioxide molecules. In CO2 however there are two double bonds and. Carbon dioxide is one part carbon and two parts oxygen.
The lone pairs push the hydrogen atoms creating a bent shape. Unlike water carbon dioxides linear structure hides the partial positive charge leaving only partial negative charges exposed on the oxygens on either end. Consider the specific heat capacities of carbon dioxide and water below.
Can you explain why they are as different as they are. Because of its bent shape. At high pressures more carbon dioxide dissolves in water.
The bonds holding the atoms together are different. Water is a bent molecule due to the lone pairs present in oxygen. Substance Specific Heat Capacity JgK Water 4184 CO2 0839 Based on the.
It can be thought of as carbon and water BUT its not structured the same as those are. It is a non-flammable gas. Both water and carbon dioxide have different critical points and triple points as well.
How might this explain why carbon dioxide and water have such different properties. Unfortunately much more water leaves the leaf than CO 2 enters for three reasons. Answer 1 of 3.
Water is polar because it has a bent geometry that places the positively-charged hydrogen atoms on one side of the molecule and the negatively-charged oxygen atom on the other side of the molecule. Though both the molecules contain carbon and oxygen the general difference between them lies in their number of oxygen of atoms carried by them. Like water carbon dioxide OCO is a polar molecule.
Silicon dioxide is network solid due to strong intermolecular covalent bond. H 2 O molecules are smaller than CO 2 molecules and so they move to their destination faster. The shapes of molecules have important implications.
How might this explain why carbon dioxide and. Carbon dioxides structure and charge distribution make it hydrophobic. As carbon dioxide CO2 has one carbon atom and two oxygen atoms while carbon monoxide CO has one carbon and one oxygen atom.
In contrast a water molecule is a polar molecule. CO2 dissolves in water when its in contact with the air and exists as a dissolved gas in water a portion of this CO2 reacts with water to form acid. Is glucose a mixture of water and carbon dioxide No.
While carbon dioxide is a naturally-occurring gas it can be harmful in highly. In carbon dioxide the molecules are bonded by weak van der Walls force. Carbonic acid a weak acid that acidifies the solution is formed when some of the carbon dioxide dissolves in the water.
Now we will study about properties and uses of carbon dioxide. While most carbon dioxide units are slightly acidic by nature the level of acidity can be modified by dissolving the molecules in water. On the other hand carbon dioxide is linear.
It is a colourless and odourless gas. It turns out that ammonia is unstable in Earths atmosphere. Carbon dioxide dissolves easily in water so our oceans absorbed much of the atmospheric CO 2 leaving an atmosphere dominated by ammonia.
Based on the chemical structure and properties of water vs. Phase Diagrams for Water and Carbon. In a water molecule there are two lone pairs of electrons connected to the oxygen.
The fact that water vapor is the dominant absorber in the Earths greenhouse effect can lead to a flawed narrative that anthropogenic carbon dioxide CO2 is. If you have really high CO2 levels in your water you can just bubble or run the water across a surface and it degasses releases some of the CO2 into the atmosphere technically removing it from the water. Hence they are molecular solids.
When the carbon atom moves along the chemical bond towards either one of the oxygen atoms or moves up and down relative to the oxygen atoms the relative distribution of the charges will be altered and hence carbon dioxide can absorb infrared radiation. Lets look at the phase diagram for carbon vs. Carbonic acid is what gives fizzy drinks their bubbles.
One is that even though both CO bonds and O-H bonds have dipoles carbon dioxide molecules are non-polar due to their linear shape and water molecules are polar due to their bent shape. Why carbon dioxide is a greenhouse gas. This is a natural gas in the atmosphere that is a byproduct of our existence on Earth specifically human and animal respiration the combustion of fossil fuels and wood fermentation and other causes.
The net effect is a partial dipole where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. The angle between bonds in water is 1045 making water a bent molecule. Explain why there may be a difference in the rate of star jumps when breathing or not breathing and why doing star jumps without breathing is unsustainable.
In carbon dioxide the carbon is. Carbon dioxide cannot form hydrogen bonds with other species of molecules. A carbon dioxide molecule consists of two oxygen atoms and one carbon atom.
Because carbon tetrachloride CCL is not soluble in water a test tube containing the two liquids will form two layers. Properties of Carbon Dioxide. Chemical properties of carbon dioxide are generally related to its level of acidity.
Sulfur dioxide SO2 and carbon dioxide CO2 have somewhat different properties in spite of the fact that both are triatomic molecules with two terminal oxygen atoms. Carbon dioxide is needed for photosynthesis to operate. It has a melting point of -556C and has a boiling point of.
A carbon dioxide molecule is a non polar molecule because of its straight line shape. Glucose is the most basic form. This process is performed in laboratories or industrial facilities because of the highly specialized nature of this work.
It is slightly toxic. Which out of CO2 and SiO2 is a molecular solid. Carbon dioxide explain why molecules of similar size and mass have significantly different specific heat capacities.
Carbon dioxide is soluble in water. It is denser than air.

What Are The Chemical Properties Of Carbon Dioxide Lisbdnet Com
Co2 H2o Carbon Dioxide Water Youtube
Carbon Dioxide Molecule Of The Month May 2012 Html Only Version

What Is Carbon Dioxide Definition Explanation Video Lesson Transcript Study Com
Carbon Dioxide Molecule Of The Month May 2012 Html Only Version

Organic Molecules Microbiology
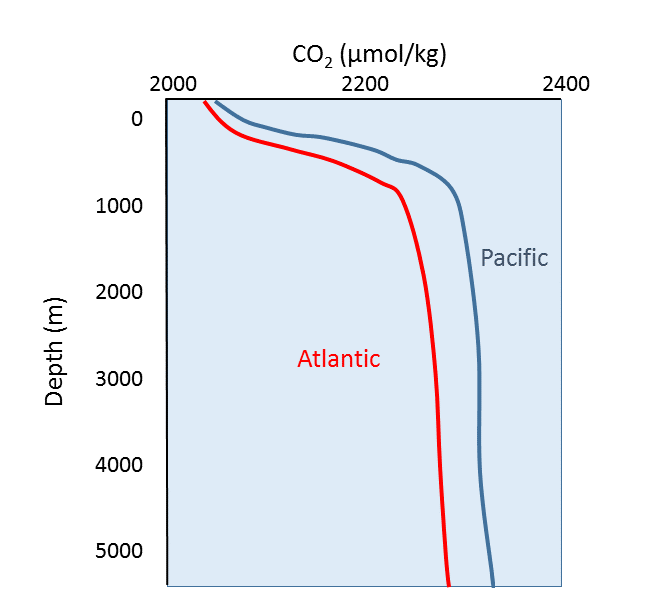
5 5 Dissolved Gases Carbon Dioxide Ph And Ocean Acidification Introduction To Oceanography

Carbon Dioxide Can Make A Solution Acidic Chapter 6 Chemical Change Middle School Chemistry
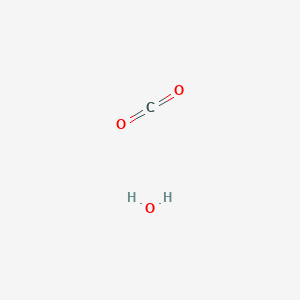
Carbon Dioxide Water Ch2o3 Pubchem
How Is Carbon Dioxide Prepared And Collected In A Laboratory Quora

Carbon Dioxide Can Make A Solution Acidic Chapter 6 Chemical Change Middle School Chemistry
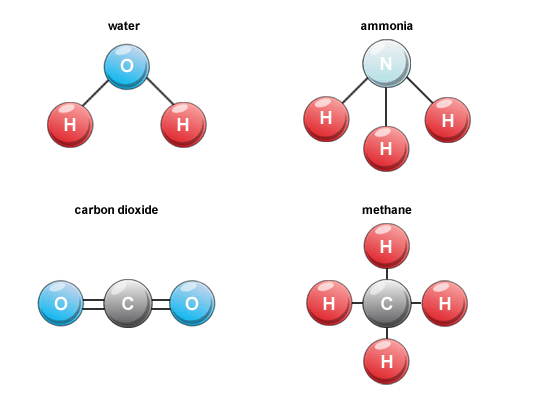
Molecule Ion Molecules Of Elements Compounds Atomicity Pmf Ias
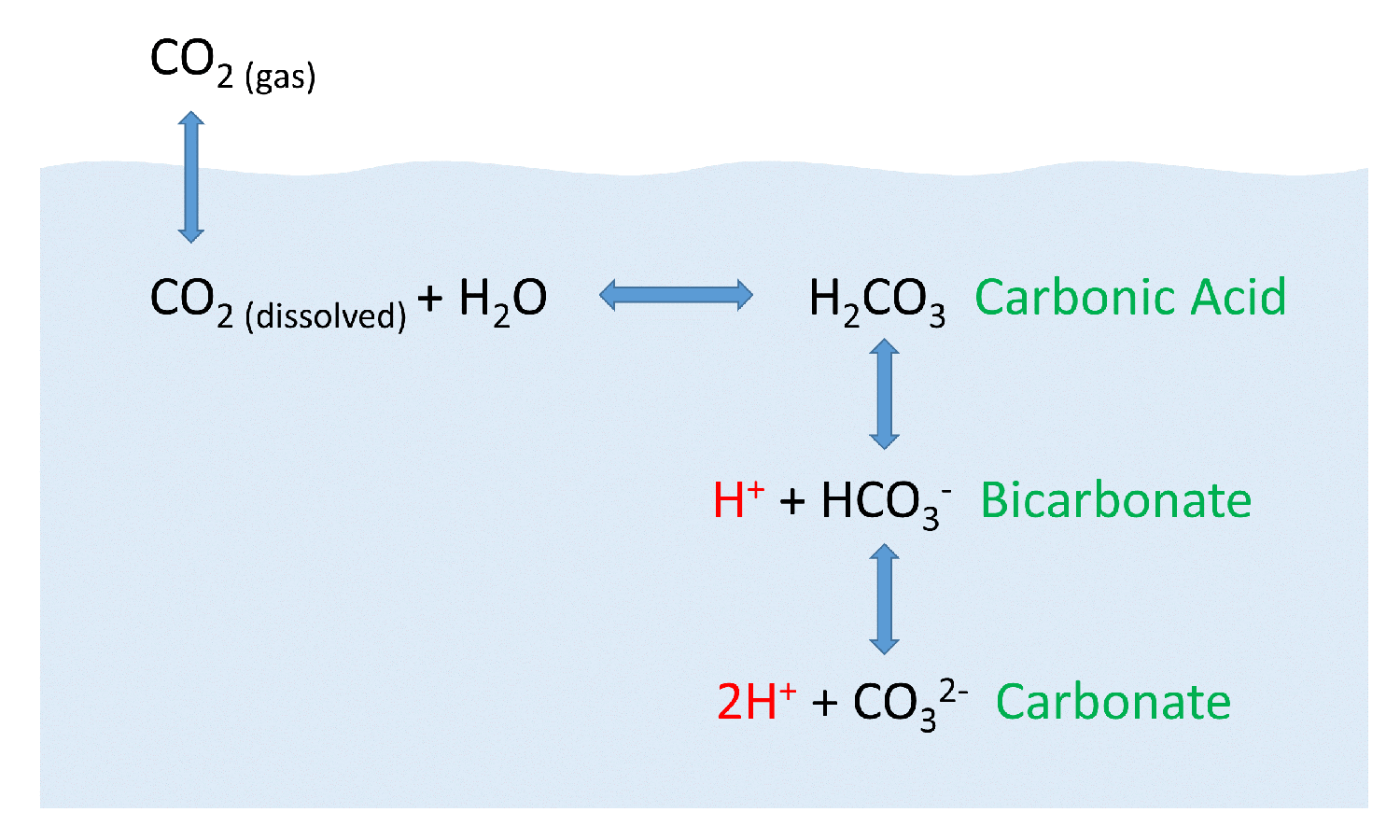
5 5 Dissolved Gases Carbon Dioxide Ph And Ocean Acidification Introduction To Oceanography

Q12 Describe The Simple Experi Lido
Carbon Dioxide Molecule Of The Month May 2012 Html Only Version
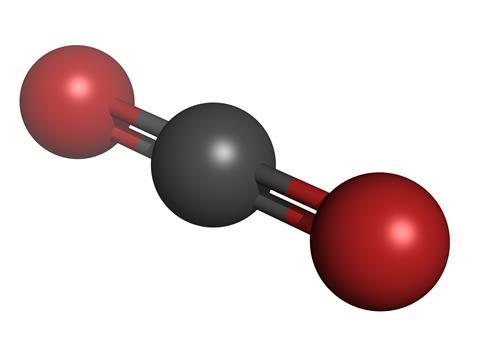
Carbon Dioxide Podcast Chemistry World

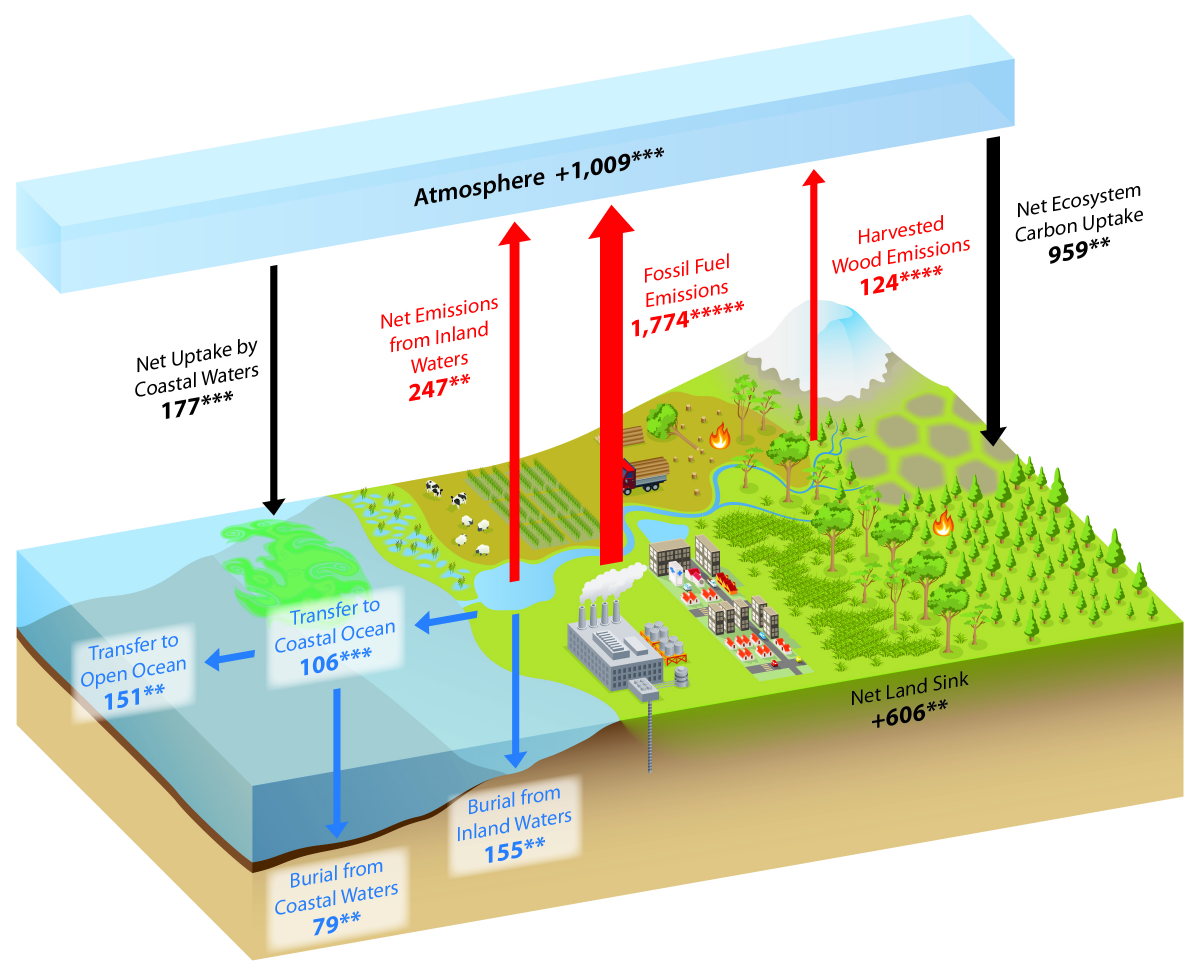
What Is The Carbon Cycle What Is The Science Behind It United States Carbon Cycle Science Program
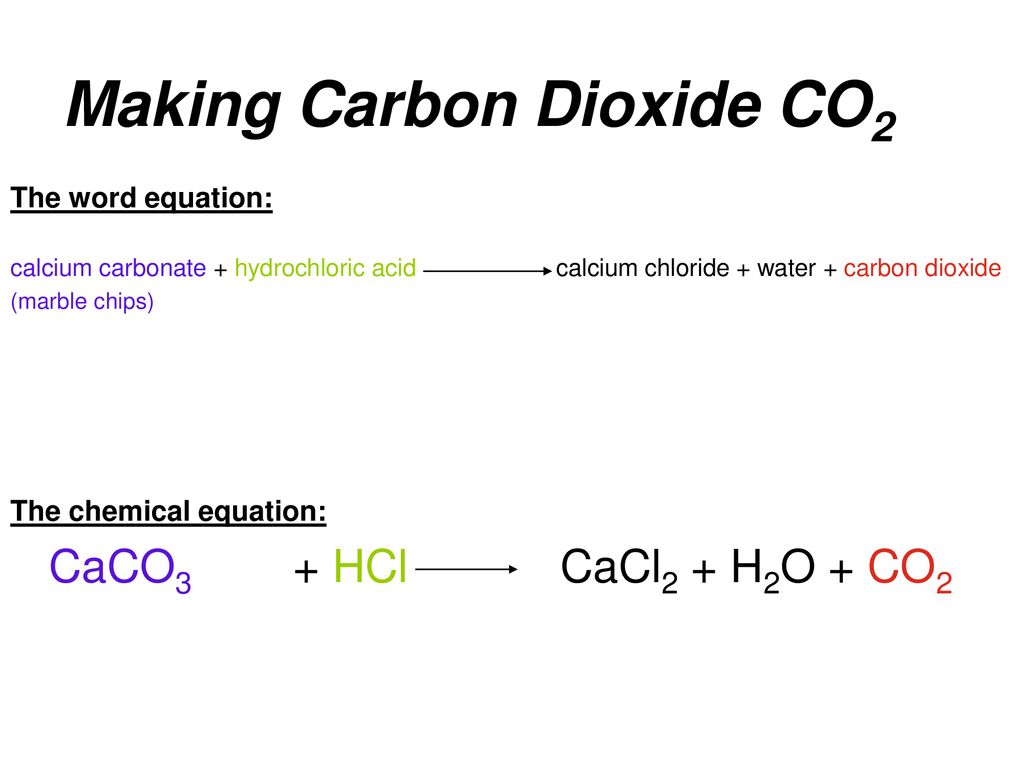
Comments
Post a Comment